Optimal blood glucose
You probably know that achieving optimal blood glucose levels is very important for lowering the risk of developing diabetes-related complications including damage to your eyes, nerves and kidneys. But having the knowledge doesn’t mean it’s easy to achieve. Joining the RADIAL trial could help.
Benefits and challenges of participating in the RADIAL trial
Benefits and challenges of participating in the RADIAL trial
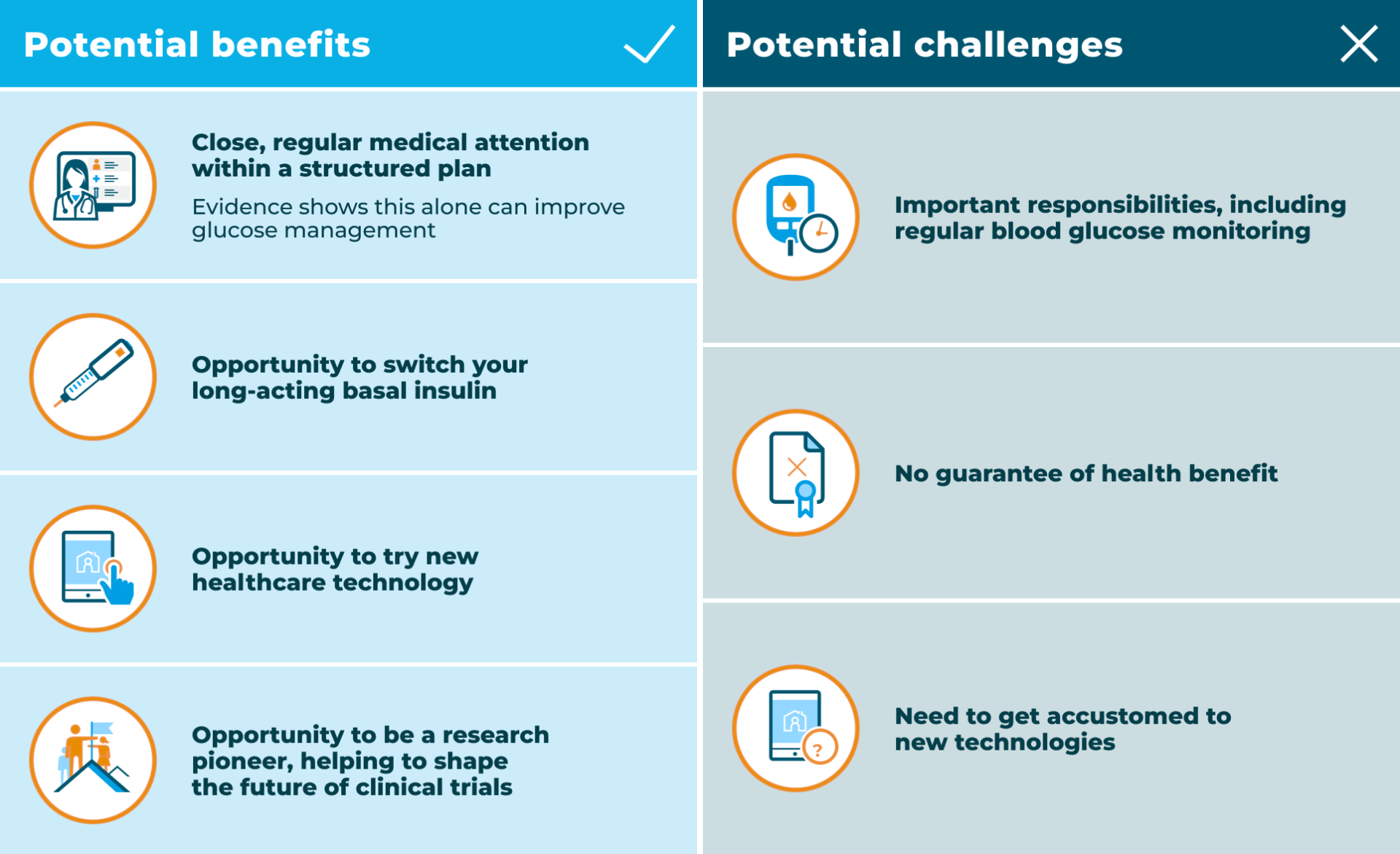
Potential benefits of
joining the RADIAL trial
Deciding to join the RADIAL trial could have a number of potential benefits:
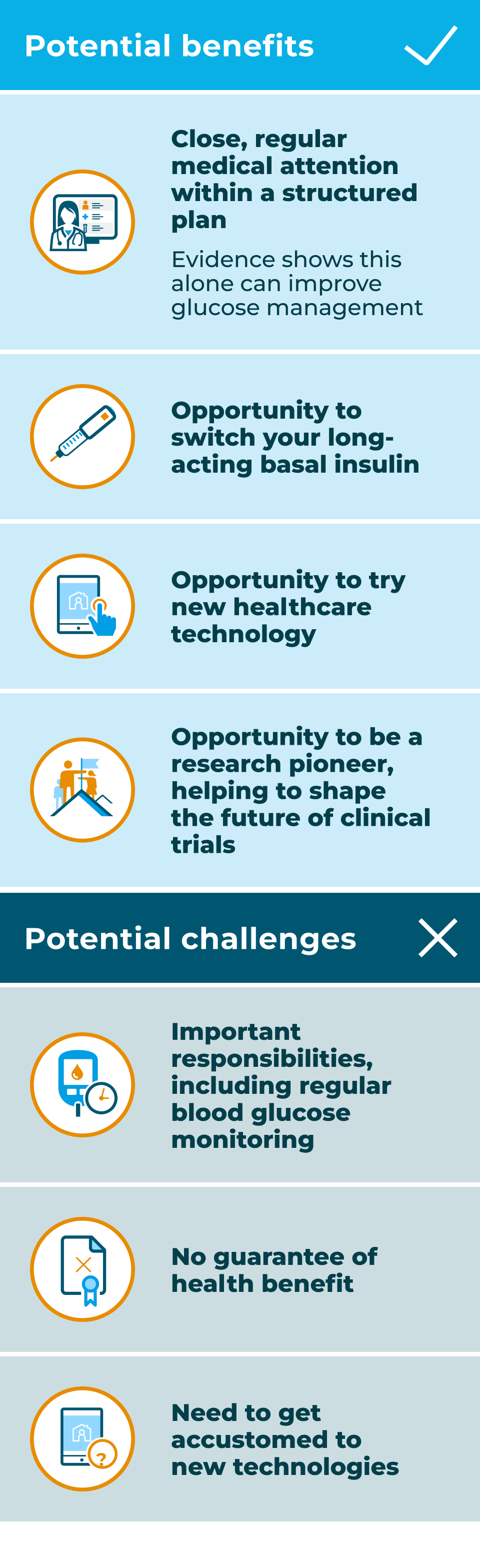
Challenges you might experience if you join the RADIAL trial
Alongside the potential benefits, you will also need to consider the potential challenges of taking part in the RADIAL trial:
RADIAL trial timeline (decentralised group)
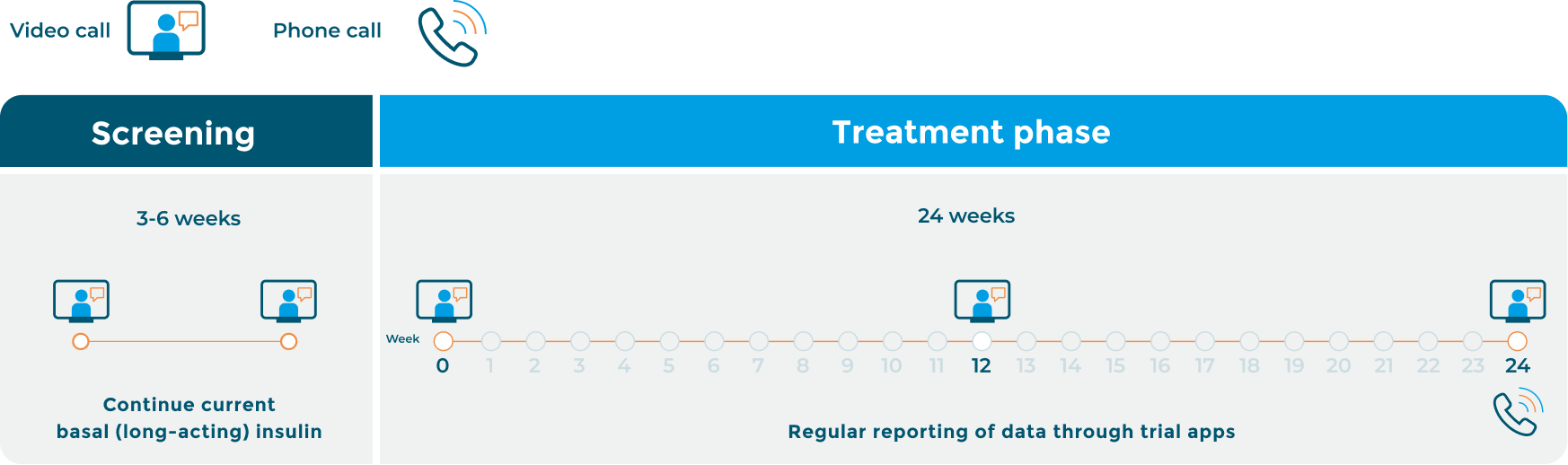
The RADIAL trial will last for about 7½ months. The first six weeks will be the screening phase where the research team will make certain the trial is suitable for you. You will continue on your current long-acting basal insulin during this period.
The main treatment period of the trial – where you will receive Toujeo® – will last for 6 months (24 weeks), with three scheduled video calls with the trial doctor.
You’ll be self-injecting Toujeo® once a day using a disposable injection pen and ultra-fine needle. Your Toujeo® pen cap will automatically record the dose, date and time each time you inject.
What you’ll need to do if you join the RADIAL trial
Testing your blood glucose regularly is a very important part of the RADIAL trial. This will be done by pricking your finger with a sterilised lancet and using a 'smart' glucometer that will automatically transfer blood glucose readings to your trial apps.
You will need to do this every morning during the time taken to find the most appropriate dose of Toujeo® and after that, three times a week. You should also do it if you suspect a hypo may be happening.
The thought of finger-pricking on a daily basis may be worrying or unappealing at first, but most people become accustomed to it fairly quickly.
Doing this is vital as it guides adjustment of your insulin dose and helps you gain the most benefit from it.
On three occasions, you will also need to provide a few drops of blood from your finger for measuring your HbA1c level (a measure of your average blood glucose over time). You will be given additional instructions on how to do this and how to send the sample to the research team.
During the RADIAL trial you will need to use trial apps to report any hypos, side effects or other medical events you may experience. The research team will contact you if there is anything of concern.
You will be given a small device for measuring your blood pressure and heart rate three times during the trial (this will be done during video calls with a trial doctor).
If you are a woman able to become pregnant, you’ll need to use a reliable form of contraception (which the trial doctor can explain) throughout the trial and take a pregnancy test at the beginning and end.
Apart from your long-acting basal insulin, all other diabetes treatments you are receiving should be continued as usual throughout the trial. You should also continue with any dietary and lifestyle advice provided by your own healthcare team.
We will ask you to complete questionnaires about your experience in the trial through the trial apps.
The responsibilities of the RADIAL trial are expected to take, on average, an hour a week and should be easy to fit into your usual day.
In addition to the scheduled video calls with the research team, medical support with any aspect of the trial will be available by phone if you need it.
Your own doctor will continue to oversee your healthcare and will be kept informed about your trial participation.
If you wish to continue with Toujeo® after the trial ends, you can discuss this with your own doctor since Toujeo® is a licensed medication in Europe.
If you decide to return to your original long-acting basal insulin, you’ll need to go through a period of dosage adjustments to get back to the best dose. This will also be needed if you wish to try a different long-acting basal insulin after the trial.
Once we know the results of the RADIAL trial, an easy-to-understand summary will be provided to you and all other participants.
It’s best if you can use your own electronic device (tablet or smartphone) to take part in the trial. For the video calls with the trial doctor, a laptop or computer (PC or Mac) with a camera (webcam) is ideal, but these can also be done on a tablet or smartphone.
If you do not have a suitable device, we may be able to provide you with a smartphone just for trial use. A quiet room will be needed for the video calls.
Apart from electronic devices, everything you need for the trial (medication and equipment) will be delivered to your home address (or location of your choice) by courier. After you receive your trial kit, a member of the research team will take you through all the contents during a video call and begin your trial training.
Further training will be provided online in a clearly structured format which you can take at your own pace. There will also be additional educational sessions with a member of the research team.
You will need to download two trial apps. These will set up automatic recording of your blood glucose readings and dosages/times of Toujeo® injections and provide access to an electronic diary (e-diary) where you’ll be able to record important trial data, join scheduled video calls with the trial doctor, receive reminders and messages, and contact the research team.
Both apps used in the trial are secure, virus-free and will respect your privacy.
Help and support with the technology will be available from the research team whenever you may need it.
- Gale EAM et al. Diabetes Care 2007; 30(12): 2989-2992
- https://care.diabetesjournals.org/content/30/12/2989.long
- Becker RHA et al Diabetes Care 2015; 38:637–643
- Riddle MC et al Diabetes Care 2014; 37(10): 2755-62
- Bolli GB et al Diab Obes Metab 2015; 17: 386–394
- Yki-Jarvinen H et al Diabetes Care 2014; 37:3235–3243

